Unlocking Chemistry Secrets: Your Guide to the Periodic Table and Valences
Remember those colorful charts on the wall in your high school science classroom? You know, the ones with the cryptic symbols and numbers? That, my friends, was your first introduction to the amazing world of the periodic table and valences (or as they say in Spanish, tabla periodica y valencias). It might have seemed overwhelming then, but trust me, understanding this chart is like unlocking a secret code to understanding how the entire universe works!
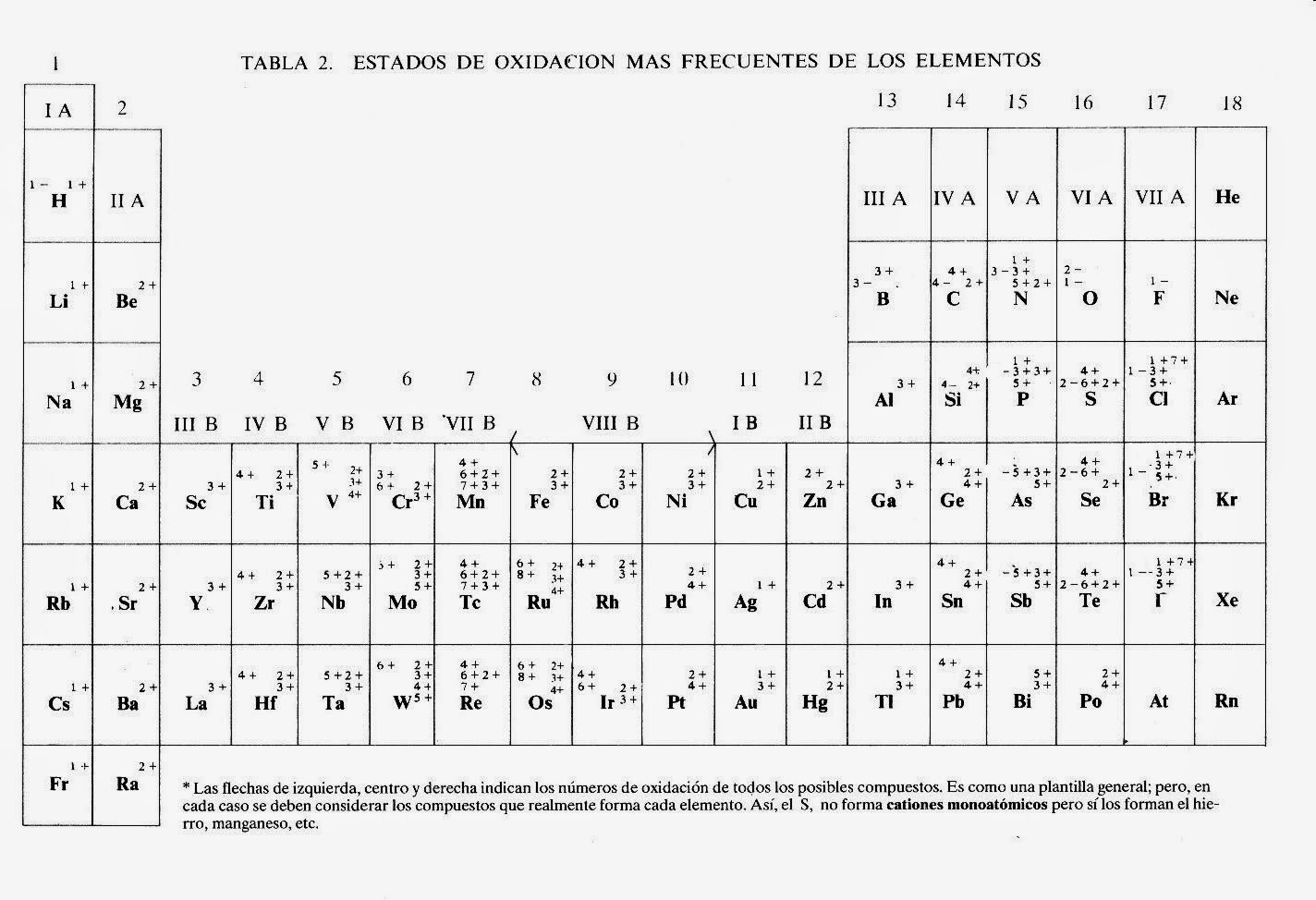
Let's break it down. The periodic table isn't just a random arrangement of elements, it's a carefully organized map based on an element's atomic number (the number of protons in its nucleus) and its chemical properties. Think of it like organizing your bookshelf: you wouldn't put cookbooks next to mystery novels, would you? Similarly, elements with similar reactivity and characteristics are grouped together on the periodic table, making it easier to predict how they'll behave in different situations.
Now, where do valences come in? Well, every element wants to be stable, like a book finding its perfect spot on your organized shelf. They achieve this stability by having a full outermost shell of electrons, and that's where valency comes in. It tells you the combining power of an element, or how many electrons an atom needs to gain, lose, or share to achieve that coveted stability. It's like a little instruction manual for how elements will interact with each other!
Understanding valency is key to predicting how elements will react. For instance, knowing that sodium (Na) has a valency of +1 and chlorine (Cl) has a valency of -1, we can predict they’ll combine in a 1:1 ratio to form sodium chloride (NaCl) - our everyday table salt. Pretty cool, huh?
Learning about the periodic table and valences might seem like a daunting task, but trust me, it’s like discovering a whole new language. It's the language of chemistry, and once you start to understand it, you'll see the world around you in a whole new light. You'll start to appreciate the intricate ways in which elements combine to form everything from the air we breathe to the food we eat. So, are you ready to embark on this exciting journey of scientific discovery?
Advantages and Disadvantages of Learning the Periodic Table and Valences
| Advantages | Disadvantages |
|---|---|
| Provides a systematic understanding of elements and their properties | Can be initially overwhelming due to the sheer volume of information |
| Helps predict chemical reactions and formulas | Requires memorization of symbols, valencies, and trends |
| Essential for further studies in chemistry and related fields | Understanding complex concepts may require additional resources and guidance |
While there are challenges to mastering the periodic table and valences, the benefits far outweigh the difficulties. With dedicated effort and a sense of curiosity, anyone can unlock the wonders of chemistry!
So, whether you're a student just starting your chemistry journey or someone who's always been curious about the building blocks of the universe, delve deeper into the world of the periodic table and valences. You'll be amazed by the hidden wonders it reveals!
The curious case of a bowling ball in an oven
Ouch my dog tore their paw pad a guide to healing prevention
Unlocking elegance the art of mastering the cursive alphabet